Drawing orbitals - HOMO LUMO
- Knowledge of the location of orbitals in luminescent lanthanides research is important. One might need to know which part of the molecule is capable of absorbing a photon - normally that is where the HOMO is located.
UV Visible Spectra of lanthanide complexes can be calculated from Sparkle Model optimized geometri
UV Visible Spectra of lanthanide complexes can be calculated from Sparkle Model optimized geometries, followed by ORCA calculations, in which the lanthanide ion is replaced by a +3e point charge.
The required citation, where this procedure has been first described, is:
The concept of a global minimum is easy to grasp. Please, consider the function below:
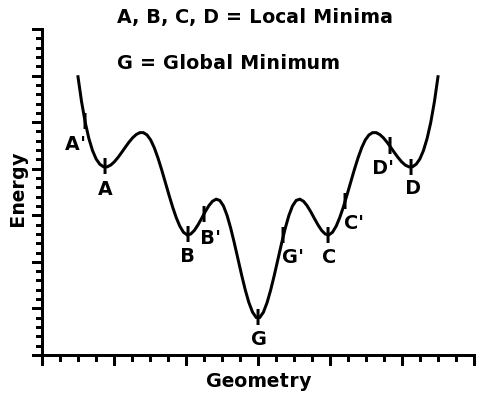
This function has five different minima: A, B, G, C, and D.
Four of them, A, B, C, and D, are called local minima because they are minima within a certain region around them.
The problem
After optimizing the geometry of a molecular system or lanthanide complex and verifying that the norm of the gradient has been sufficiently reduced, users tend to be satisfied with the geometry obtained and proceed to compute other properties.
Is this warranted?
No!
Actually it is very important to determine if this geometry corresponds to a true minimum in the potential energy hypersurface where the atoms move.
How?
The solution